A big opportunity and even bigger constraints
Existing solutions preventing injury can be unreliable, difficult to use and potentially hazardous. So when Unilife had the opportunity to develop a safer pre-filled syringe, for a leading pharmaceutical company, we were eager to support them on the journey.
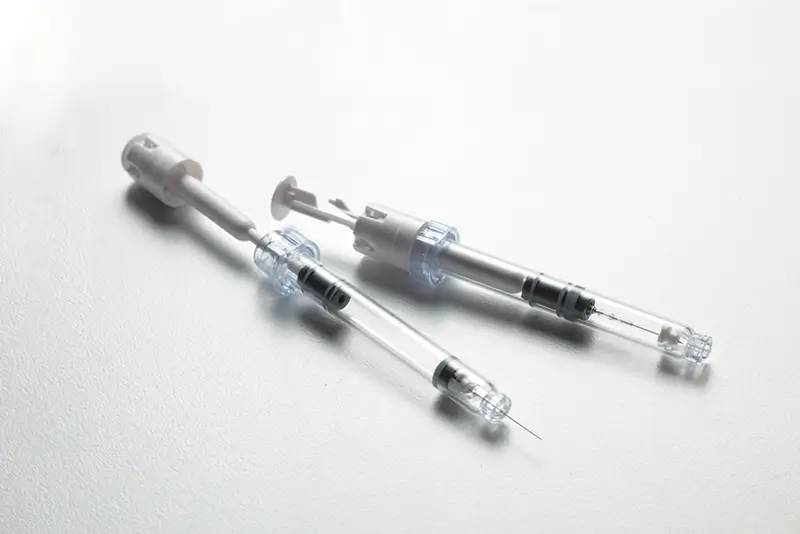
The challenge was clear, create the world’s first prefilled syringe with integrated safety, without affecting the filling process, drug or its use. We had to show it could be done to pharma standards and that we were the ones to do it.
Breaking down what we knew to fundamentals enabled us to explore and identify new options, but we missed the mark. The constraints were bigger than anticipated. So we had to dig in and question everything, the rethink started on the plane home.
Creating was not enough, prove it!
The recipe for producing prefilled syringes is well mastered by a few large organisations and they were all nervous at the thought of the smallest change.
So focusing on a new syringe design was not enough. We had to create new methods of predicting performance and simulating automated production processes. We had to prove it was feasible, viable and desirable on a large scale, or no deal.
More than just a new device
The complexity of the challenge was met with a simple device, new technology and new intellectual property, whilst not threatening constraints not up for negotiation.
The result? A record licensing deal, a move to the USA, listing on the NASDAQ, more people employed, an organisation funded and capable of developing more meaningful products and a safer device for those who care for us.