Complexity and compromises, yet little innovation
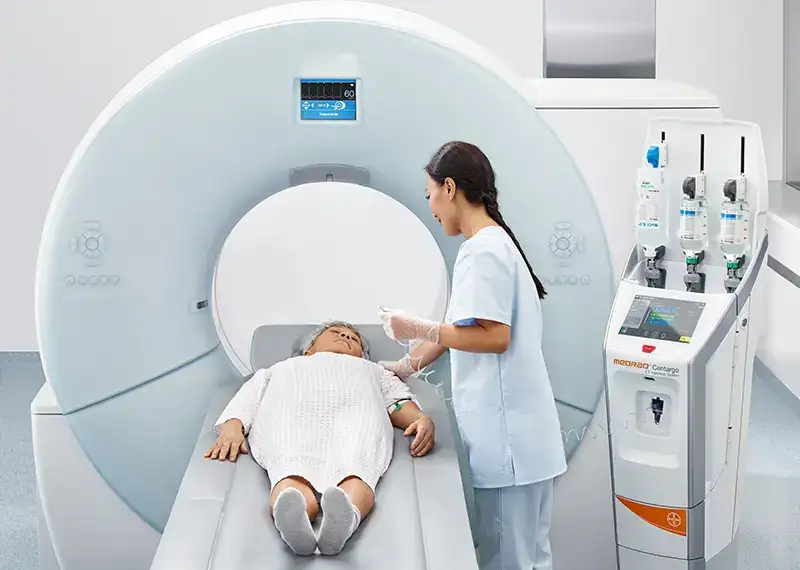
Ocular injections utilise devices not designed for the unique challenges of the eye. This introduces significant variability in drug delivery and procedural complexity, resulting in unnecessary risks and workflow inefficiencies. With little innovation to date, rising incidents of ocular disease and growing workforce shortages, the issues are exacerbating.

The status quo results in issues for all involved
The emerging standard of care utilises glass prefilled syringes in an attempt to improve workflow efficiency. Whilst such syringes are a fixture in drug delivery generally, their use in ocular drug delivery leads to detrimental compromises. They do not fully address workflow issues and are generally unsuitable for emerging novel drug formulations.

Introducing

Enabling and enhancing therapeutic efficacy, procedural simplicity, and workflow efficiency in a single device.
Product Specifications:
*Based on internal testing, data and analysis
A comprehensive new standard of care

Benefits
With OKO, achieving efficiency, safety, and exceptional patient care simultaneously is possible.
*Based on internal testing, data and analysis
Impact
OKO is a comprehensive drug delivery device that brings benefits to all stakeholders of injectable ocular therapies.
Suitable for various applications
OKOTM is a versatile platform capable of delivering current and novel therapies for in-clinic, non-surgical ocular injections.
Disclaimer
OKO(TM) Ophthalmic Drug Delivery System is not yet commercially available. The information on this website represents the current state of development and is for demonstration purpose only. Users accessing this website acknowledge and agree that information presented may not comply with local laws, regulations, or product registration requirements in their country or region. The company disclaims all liability for any actions taken based on information provided on this website.
OKO and IDE Group are trademarks of I D & E Pty Ltd.
Footnotes
[1] Vita L. S. Dingerkus, Gabor M. Somfai, Stephan Kinzl, Selim I.Orgül, Matthias D. Becker, Florian M. Heussen, “Incidence of severe rise in intraocular pressure after intravitreous injection of aflibercept with prefilled syringes,” Nature, Scientific Reports, (2022)
[3] Eleni Karatsai, Shruti Chandra, Sandro Fasolo, Sobha Sivaprasad, Robin Hamilton, “Prefilled Eylea Syringe: our recent experience,” Springer Nature, The Royal College of Ophthalmologists © Crown (2021)
[4] Sean T. Berkowitz MD, MBA , Avni P. Finn MD, MBA, Ravi Parikh MD, MPH, Ajay E. Kuriyan MD, MS, Shriji Patel MD, “Ophthalmology Workforce Projections in the United States, 2020 to 2035” Ophthalmology, Vol 131, Iss 2 (2024), Pages 133-139
[5] Timothy G Murray, Paul Tornambe, Pravin Dugel, Kuo B. Tong, Evaluation of economic efficiencies in clinical retina practice: activity-based cost analysis and modeling to determine impacts of changes in patient management,” Clinical Ophthalmology, 5 (2011) Pages 913–925
[6] Mali Okada, MMed, Paul Mitchell, PhD, Robert P. Finger, MD, PhD, Bora Eldem, MD, S. James Talks, MRCP, FRCOphth, Ceri Hirst, PhD, Luciano Paladini, MD, Jane Barratt, MSc, PhD, Tien Yin Wong, MD, PhD, Anat Loewenstein, MD, “Nonadherence or Nonpersistence to Intravitreal Injection Therapy for Neovascular Age-Related Macular Degeneration” American Academy of Ophthalmology, Vol 128, No 2, (2020) Pages 234-247