The Partnership
When Bayer approached us to collaborate on developing a new generation of contrast injectors, we were already forming a close partnership through some discreet project work. Good working partnerships, where we put each other’s interests at heart, are essential to understanding what is critical. They also allow us to leverage each other’s strengths and supplement each other’s weaknesses. Something that was needed to tackle a market threat that was becoming an issue for Bayer.
The Challenge
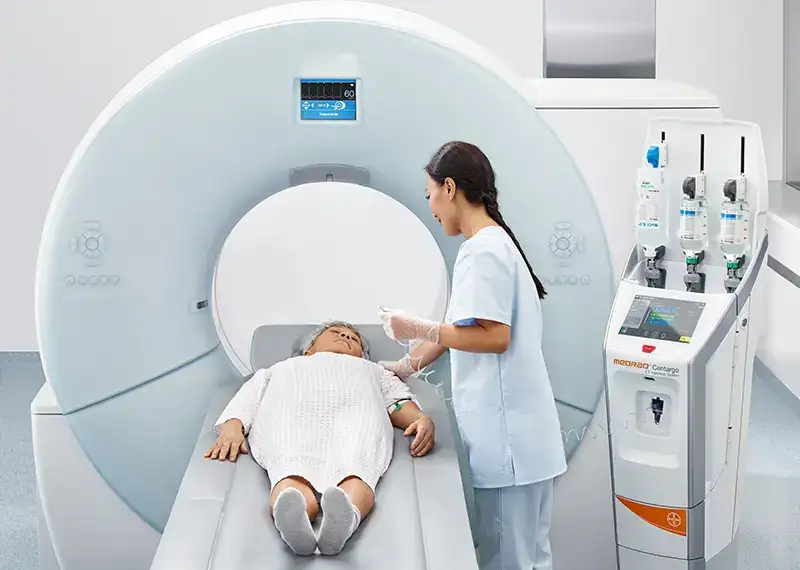
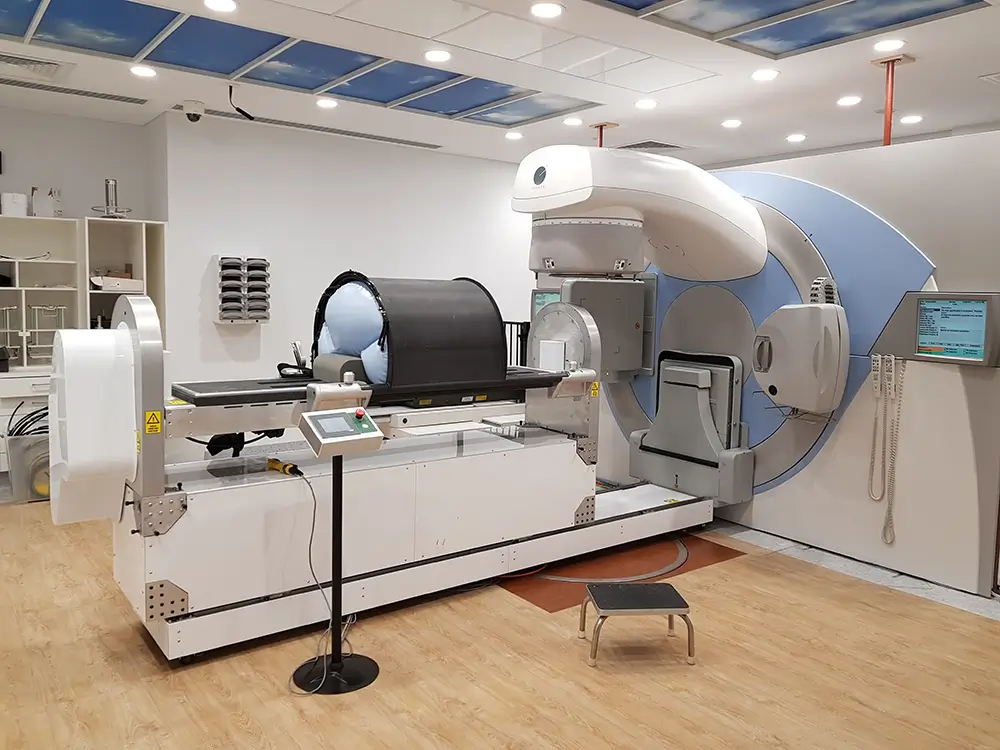
Most CT injector systems traditionally require a high level of manual interaction by the radiologist. They have to set up the injector, connect consumables and fill syringes with contrast that is then injected into the patient being scanned. This takes critical time and attention away from the patient and provides quality images for effective diagnosis.
However, change was on the horizon. Bayer observed a new trend with the emergence of new multi-use contrast injectors. These injectors reduced the burden for radiologists in setting up an injector, allowing them to scan multiple patients with less effort. Although the clear market leader, with best-in-class injectors, Bayer did not have such an offering, their market share was now being threatened
Digging Deeper
Bayer’s competitors created products based on perastaltic pump technology. It seemed the logical choice to ensure contrast is available at any time and reduce handling steps. However, it comes at a cost and sacrifices some performance. Instead of replicating this approach, we followed our curiosity and discovered a superior product. To better understand the project, we spent time with the key stakeholders, conducted interviews, gathered insights and analysed what we found. We visited multiple CT suites across the country to explore the process and competitors and understand Bayer’s business. This provided us with valuable insights into the current challenges and opportunities.
The critical insight we discovered was that radiologists did not require contrast at any time. Instead, they needed it only when ready to inject. Reframing the problem this way enabled us to envision a new generation of syringe-based systems that gives radiologists the same benefits without compromises. It also allowed Bayer to leverage their 30-year expertise in syringe-based injector systems to tackle the market trend on their terms. An approach many thought to be impossible.
The newly envisioned system would enable higher pressures for more injection protocols, smoother fluid transitions and an efficient set-up process in less than 2 minutes. This would result in a unique outcome, improving the diagnostic imaging whilst limiting the risks of cross-contamination. It would also minimise the intervention of the radiographer and allow them more time for the patient.
The Solution
Our product development strategy was to ensure the product was desirable to its users and stakeholders, technically feasible and safe, as well as commercially viable. Our plan took an iterative approach to reduce risk and remove uncertainty whilst building value in the solution as quickly as possible. The key in this approach was a fully integrated cross-functional team working closely together to develop all system elements. Despite the geographical dispersion of our team in Sydney and Bayer’s teams in Pittsburgh and Berlin, we directed our energy towards our common purpose. We embraced innovative solutions to drive the best ideas forward.
The process involved the creation and in-depth testing of several prototypes and created a suite of unique IPs. The IP created was vital for Bayer, enabling them to invest in this project confidently to achieve their commercial goals. To make a safe and regulatory compliant device, we were working together through the details necessary. Our partnership took us from project inception, detailed design and manufacturing to the first installed units.
Our Executive Director shared his insights on the project and its outcomes:
It is award-winning for sure, but the real award is the impressive demand for the product, which is truly inspiring. To be honest, it is hard not to get emotional about this project knowing all the effort and passion we as a united team put into it and the challenges and worries we overcame. The overwhelmingly positive feedback from the market is truly important to assure us we are achieving our mission of creating a better future.
Better for clinics and hospitals, in doing more for less. Better for the radiologists to allow them to focus on the patients and get an accurate diagnosis for better treatment. And better for our partners and friends at Bayer, supporting more sales, more jobs, and higher returns for their shareholders. Not to mention what it meant for IDE. You really can’t ask for more than all that.
The final product marked the beginning of Centargo: a unique and novel CT injector. The end users love the product based on stellar early feedback and high global demand. Together with Bayer, we not only successfully invented a novel product but changed the face of injectors to come. To the benefit of the radiology departments worldwide and, ultimately, the patient.
We started working with IDE very early on in this project. Their commitment to being a full partner in development meant that they now have a nearly equivalent understanding of our customers needs to the team at Bayer Radiology.
This commitment led to a product that really does what the clinician expects: IDE brought product development, industrial design and fundamental engineering professionals to the table to complement Bayer’s expertise and result in a product where everyone is invested in its success.
– Mick Brooks
Head of Bayer Radiology R&D Software Development, Bayer Healthcare