At IDE, we understand the critical role software plays in the medical device ecosystem
Our team of experts is proficient in usability engineering, industrial design, ergonomics, mechanical and electronics engineering, embedded firmware, mobile software applications, web services and cloud development.
From Software Tools and Prototypes that enable medical devices (hardware), to Software as a Medical Device (SaMD), solutions that inform clinical decisions and provide direct therapy, our expertise spans the entire digital health spectrum;
Why Choose IDE?
Collaborating closely with our Quality and Regulatory teams, we work with you to develop the most effective pathway to commercialisation:
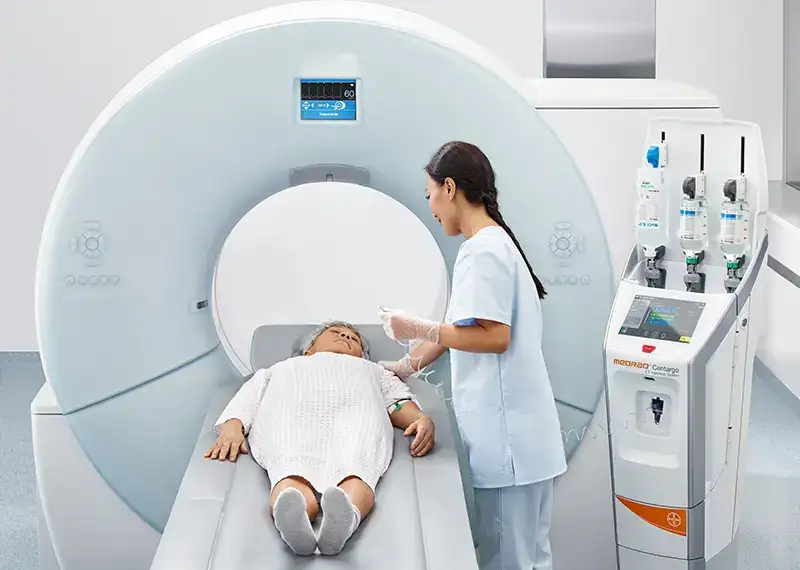
On the Bayer MEDRAD Centargo CT Injector system, IDE has shown that they truly care about UX and user-centered design. The passion for what is right and good has meant that Centargo has a very carefully thought out and principled UX design. I cannot emphasize enough how truly grateful I am that I engaged with IDE despite the time zone and geographical differences. Wherever you are, it’s worth it, just do it, and work with IDE on your UX.
– John Volkar
Centargo Software Lead and Chief Architect, Bayer Healthcare